Our Products - Specialization
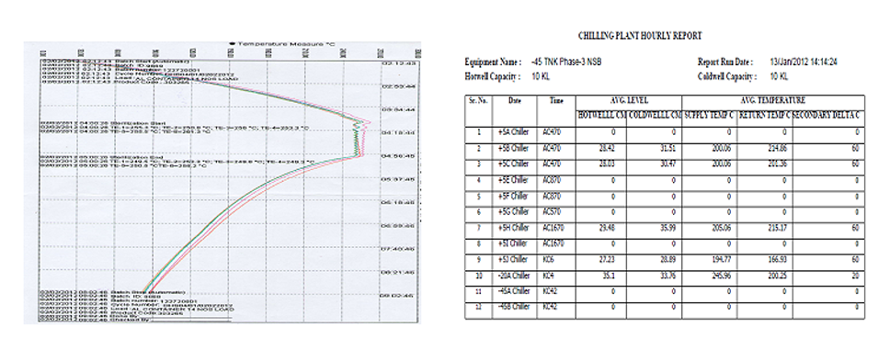
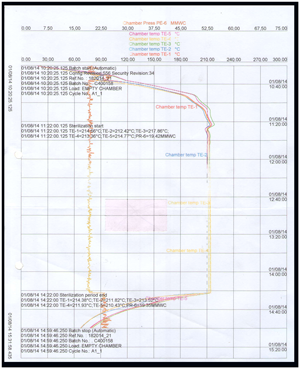
Durend Controls has specialization in report generation according to 21CFR Part 11. This code of federal regulations deals with the Food & Drug Administration (FDA) guidelines on electronic record & electronic signatures in the United States, part 11, as it is commonly called, defines the criteria under which electronic record and electronic signatures to be trustworthy, reliable and equivalent to paper records. It includes validation of instruments, maintenance of electronic records, electronic copies of electronic records, etc.
Under these codes, we are prominent service provider. To work under these codes requires high level of accuracy and we are master in this. We provide customized report following codes of 21CFR Part 11 to various customer including pharmaceuticals.