

Aquatic Solutions has special division focused on Pharmaceutical & Healthcare industry. These industries require special attention, as the world standard of water quality used keeps updating.
Our mission is to provide systems that have excellent results & low costs leading to savings.
Active implementation of good quality is our priority. Water Systems are designed in accordance with the requirements of FDA, cGAMP, cGMP, cUSP, & cPH Eur.
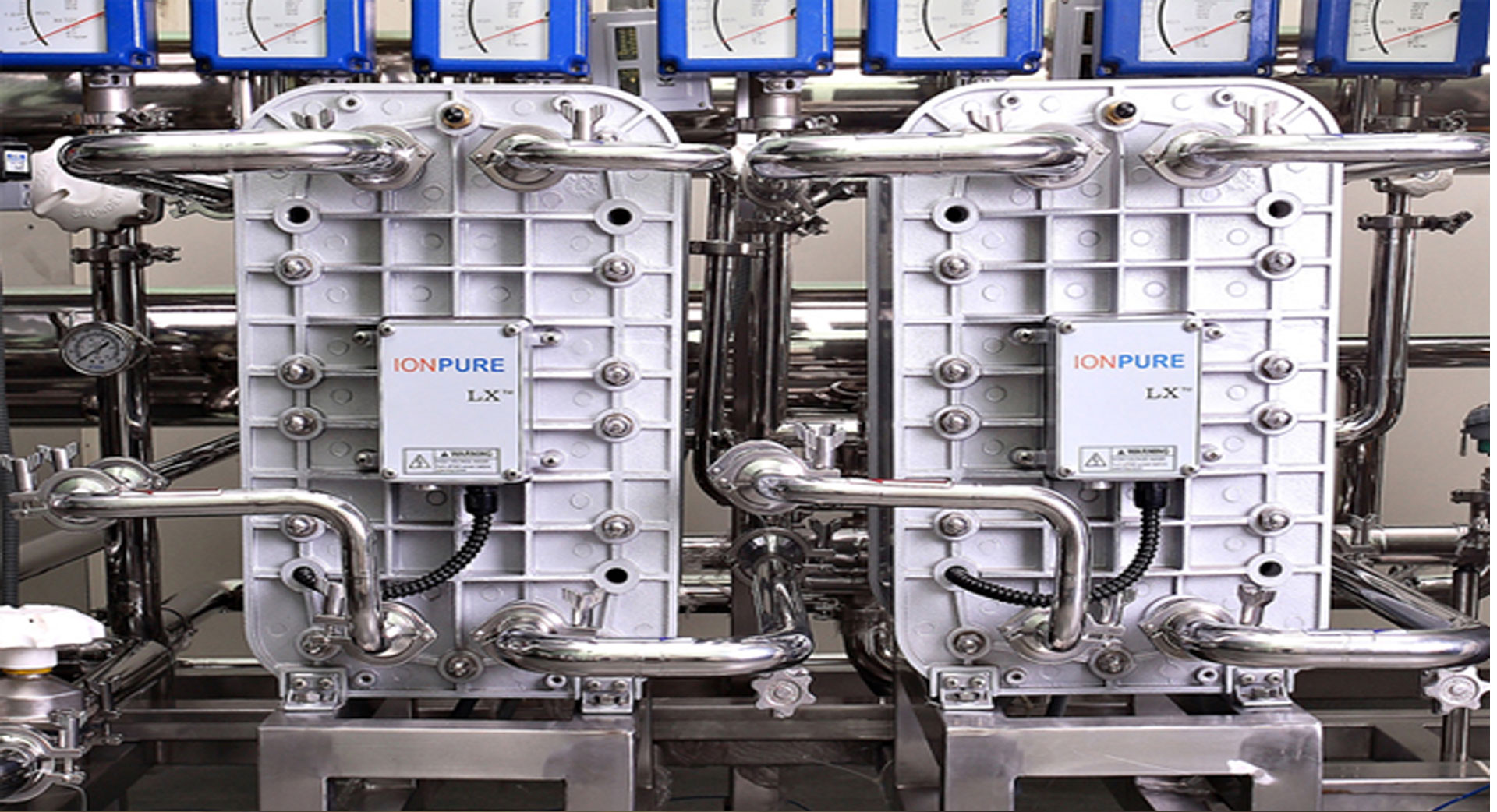
Salient Features of Aquatic Solutions Purified Water Generation Systems
- Systems conforming water quality according to USP norms for Pharma use.
- Avaliable over a wide range of flow with Fully Automatic(PLC) or Semi Automatic options.
- Hot water Sanitization & Ozone Sanitization Facility.
- High Quality equipments, Complete Orbital Welding.
- Complete with all monitoring & controlling instruments
In order to meet the PW & WFI quality objectives, the USA FDA recommends generation of PW & WFI "where & when" required. However, with growing demand, bigger plant size & layouts for Pharmaceutical & Biotech industries becoming difficult PW & WFI "where & when" required. Our Water Storage & Distribution Systems are designed to store and distribute PW & WFI water to various user points maintaining the generated water quality.
Salient Features of Aquatic Solutions RO Systems:
PW & WFI Storage Tanks- All Tanks designed & Manufactured as per ASME SEC VIII, DIV I.
- SS316L Wetted Parts with electro-polished upto 0.4 Ra finish, Outer Insulation & Cladding.
- Third Party Riboflavin tested.
- Accessories like Dynamic Spray ball, sanitary level sensor, compound pressure gauge.
- Heater & Electrical heat traced vent filter housing with 0.2 micron hydrophobic vent filter.
- Fully drainable Sanitary Pumps selected to ensure minimum velocity of 1.2 m/s
- Provided with VFD & interlocked flow transmitters in the return line.
- Swing arm assembly for easy pump changeover.
- Instrumentation to monitor Flow, Pressure, Temperature, TOC & Conductivity.
- Sanitization of Tank & Loop by Ozonation.
- SS316L Electro polished laser welded bead crushed rube as per ASTM A270/269.
- Dead Lag not more than 1.5D.
- Zero Dead Lag block valves with inbuilt sampling port for point of use.
- Minimum gradient of 1:100 for self drain ability.
- Complete orbital welding & inspected by boroscopy machine.
- Heat Exchanger provided to cool PW & WFI as per the process requirements.
Aquatic Solutions provides documentation backed with necessary test & calibration certificates with full NABL traceability. For pharmaceutical & biotechnology industry the documentation is done to ensure proper regulatory compliance DQ, FAT, IQ SAT, OQ & PQ
The documentation package is well designed & organized to provide validated information on the project life cycle from design stage to site to acceptance test. Our dedicated validation team Provides a comprehensive validation support to the customer including validation protocol preparation, site tests activities, instrumentation calibration & validation reports organization.
You will take comfort in knowing that our engineers are the most connected and tech savy in the industry. We pride ourselves in our ability to deliver results. Our technology platform helps ensure we deliver those result consistently & reliably.
